Bcs Classification Of Drugs List
The Biopharmaceutical Classification System (BCS) has been a prognostic tool for assessing the potential effects of formulation on the human drug oral bioavailability. When used in conjunction with in vitro dissolution tests, the BCS can support the prediction of in vivo product performance and the development of mechanistic models that support formulation assessments through the generation of “what if” scenarios. To date, the applicability of existing human BCS criteria has not been evaluated in dogs, thereby limiting its use in canine drug development. Therefore, we examined 50 drugs for which absolute bioavailability ( F) was available both in dogs and humans. The drugs were also evaluated for any potential association between solubility (calculated from the dose number, Do) or lipophilicity (LogP) and F in dogs.
In humans, solubility is determined in 250 mL of fluid. However, the appropriate volume for classifying drug solubility in dogs has not been established. In this analysis, the estimated volume of a water flush administered to fasted dogs (6 mL) and a volume of 250 mL scaled to a Beagle dog (35 mL) were examined. In addition, in humans, a Do value greater than 1.0 is used to define a compound as highly soluble and a LogP value greater than 1.72 as high permeability.
These same criteria were applied for defining highly soluble and highly permeable in dogs. Whether using 35 or 6 mL to determine Do, the canine solubility classification remained unchanged for all but seven compounds.
There were no clear associations between a drug’s F in dogs and humans or between the canine value of F and either its human BCS classification, its LogP value, or the canine Do estimate. There was a tendency for those drugs with canine values of F equal to or greater than 80% to have LogP values equal to or greater than 1.0.
Exceptions to this observation tended to be those compounds known to be absorbed via mechanisms other than passive diffusion (e.g., via transporters or paracellular transporters). Although there are limitations to the approach used in this study, the results of our assessment strongly suggest that the human BCS classification system requires substantial modification before it can be reliably applied to dogs. INTRODUCTION The United States Pharmacopeial Convention (USP) authorized the creation of an advisory panel to investigate the possibility of applying the principles of the Biopharmaceutics Classification System (BCS) to veterinary drugs—specifically, solid oral formulations administered to dogs. Developed for human pharmaceutical compounds (–), the BCS is an important tool that facilitates product development and regulatory decisions.
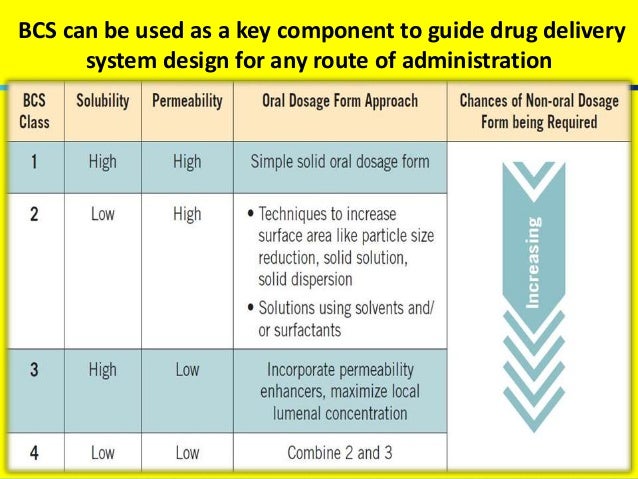
By understanding the solubility of a compound in biorelevant media and its permeability across biological membranes, the rate limiting factors determining the rate and extent of oral drug absorption can be identified. This information can be invaluable for predicting the potential influence of formulation and physiological variables on oral drug bioavailability. Within the framework of human pharmaceuticals, drugs can be classified into one of the following four BCS categories:.
Class IV: low solubility, low permeability: very poor oral bioavailability A complementary classification system was proposed by Wu and Benet (, ). They recognized that drugs exhibiting high permeability are generally extensively metabolized, while poorly permeable compounds are primarily eliminated as unchanged drug in the bile and urine. Thus, the Biopharmaceutical Drug Disposition Classification System (BDDCS) has been used to predict drug disposition and potential drug-drug interactions in the intestine and the liver and potentially the kidney and brain. Although the solubility criteria for the BCS and BDDCS are the same, there is a substantial difference in the second variable being considered. For the BDDCS, the second classification is related to the extent of drug metabolism. Conversely, the assessment of permeability in the BCS is linked to the extent of intestinal absorption, i.e., a drug is considered to be highly permeable when the extent of the systemic absorption (parent drug plus metabolites) in humans is determined to be at least 90% of an administered dose based on a mass balance determination or in comparison to an intravenous reference dose.
Accordingly, the BCS and BDDCS classification of a drug may differ. The US Food and Drug Administration’s (FDA’s) Center for Drug Evaluation and Research (CDER) has incorporated BCS concepts into guidance documents for human medications into the 2000 FDA Guidance for Industry, including guidance for the waiver of in vivo bioequivalence study requirements for high solubility/high permeability drug products. However, the BCS has not as yet been extrapolated for application to veterinary drugs.
The reason for this gap is that the BCS was developed based upon human digestive physiology, which can be vastly different from that observed in veterinary species. Converter unable to obtain hardware information. Given the similarity of therapeutic entities used in the dogs and humans, and because of the use of the dog as a preclinical species for human medicine , it would be of particular value to have an understanding of how the BCS criteria can be translated between human and canine gastrointestinal (GI) physiologies.
The solubility criteria used both by the BCS and the BDDCS rely upon formulation considerations in that it is based upon the highest dose that will be administered. Both the BCS and the BDDCS define a high solubility compound at the highest marketed dose strength that is soluble in 250 mL of water over the pH range of 1–7.5 at 37°C. This definition differs from that of “intrinsic solubility,” which reflects the equilibrium aqueous solubility of a compound. For acids and bases, intrinsic solubility represents the concentration of the unionized species in a saturated solution at the pH value where that compound is fully unionized. While there has been some debate regarding certain compounds whose intrinsic solubility may not be accurately defined when using conventional media (, ), those considerations are founded upon the perspective of a drug’s physicochemical characteristics rather than on the in vivo conditions into which that drug will be introduced. Thus, while intrinsic solubility is solely a function of the molecule, the BCS (or BDDCS)-based solubility criteria is dependent upon physiological conditions and the corresponding targeted therapeutic dose. Unfortunately, what constitutes the BCS-based criteria for high or low solubility is currently undefined for dogs because of complexities associated with interspecies differences in the composition of the GI milieu.
Another obstacle confronted when trying to establish canine-specific BCS criteria is the challenge associated with the classification of intestinal permeability. Despite the range of high throughput systems available for examining human intestinal permeability, such as Caco-2 cells, parallel artificial membrane permeability assay (PAMPA), and phospholipid vesicle-based permeation assay (PVPA), these methods for estimating drug permeability have only been applied to human drugs (, ). These systems have not been developed and validated for application to drug permeability across the canine intestine (, ). Moreover, while one may argue that transcellular permeability should be similar in humans and dogs, the GI tract of the dog tends to be more permeable (leakier) because of the larger intercellular pores.
Currently, the existing in vitro methods for evaluating drug permeability have not succeeded in providing data that can be extrapolated to dogs. For this reason, we needed to resort to comparisons based upon the use of absolute bioavailability. Because there are no suitable in vitro methods to assess effective permeability in dogs (Peff), we have used absolute bioavailability ( F) for this analysis. We have justified this approach because a comparison of drug absorption across human colonic epithelium cell layers (Caco-2 cell line) to absorption across canine colon tissue did not show a relationship. The Ussing chamber technique, which has been evaluated for other veterinary species , has not been applicable for canine studies because of the fragility of the tissue. Membrane damage that occurs prevents permeability measurements using this technique in dogs. Therefore, without the availability of these in vitro tools, other data must be used to predict permeability and apply BCS criteria for oral drugs administered to dogs.
The current investigation was undertaken because of the lack of established BCS criteria to evaluate oral medications administered to dogs. The objectives were to examine the properties that define human BCS criteria for drugs and to compare this information to pharmacokinetic data available from studies in dogs.
Without in vitro intestinal permeability data in dogs, another parameter must be considered to classify a drug as either high or low permeability. To this end, there are numerous molecular factors that impact drug transcellular permeability, including hydrogen bonding properties, molecular size and shape, polarity, flexibility, and ionization properties (, ). There is no “gold standard” or even a suggested criterion based upon the log of the lipophilicity coefficient (LogP) pH-dependent lipophilicity value (LogD) that has been proposed as a cutoff value.
Therefore, we focused on the use of the systemic absorption value (absolute bioavailability, F), which we were able to obtain from the published literature. It was assumed that if the value of F is high, permeability (via active or passive processes) must likewise be high. We also acknowledged at the outset that the converse was not necessarily true and that the use of F as an indicator of drug permeability will produce some false negative results, i.e., there are drugs that have high permeability, but low values of F because of other factors such as intestinal efflux or high first-pass metabolism. A drug solubility classification has not been established for medications administered to dogs (, ). To classify a drug as either high or low solubility in dogs requires that one knows the ideal volume in which to measure solubility. In this study, we have examined two different volumes and considered whether this parameter can be useful to predict oral absorption of medications in dogs. Ultimately, the objective of this study was to identify the in vitro drug properties with respect to their potential impact on dog-human differences and similarities on oral drug solubility and permeability.
The foundational assumption was that if properties can be identified, we could then generate dog-specific criteria for applying BCS concepts to understand the critical formulation and physiological variables that can influence canine oral drug absorption. Similar to its tremendous influence on human drug product development and regulatory evaluation, a roadmap for screening oral product formulations, if applied to veterinary drug products, would provide a tool for screening new formulations.
Additional benefits that would be associated with a canine-specific BCS would be an improvement on our ability to compare human formulations for potential testing and clinical use based upon information obtained in dogs (and vice versa). One of our objectives was to extend our assessment beyond the results reported by Chiou et al.in which the oral bioavailability of 43 compounds was compared in humans and dogs. In that study, they observed that while 22 of the 43 compounds were completely absorbed in both humans and dogs, the overall interspecies correlation of F values was low (coefficient of determination, R 2 = 0.51). A pitfall associated with the investigation by Chiou et al. Was that much of the data were based upon radiolabeled data, thereby precluding a differentiation between parent compound and metabolites. Given the potential for interspecies differences in intestinal metabolism, and since F values were based on total urinary recovery of radioactivity of the drug (thereby further confounding the comparison with potential differences in post-absorption processes), we could not use that information to generate predictions on the permeability component of the BCS.
However, we also recognized that a drug with a high first-pass effect may be reported with high F in the study by Chiou et al. , but not in our study reported. Thus, in addition to evaluation of BCS classification versus F in dogs and humans, we compared our F values with those reported by Chiou et al.
EXPERIMENTAL METHODS To explore the potential application of BCS principles for oral drugs administered to dogs, pharmacokinetic, lipophilicity, and solubility data were either calculated or were obtained from existing literature. Do = M V C where M is the dose strength of the tablet/capsule, V is the volume administered (defined as 250 mL for humans), and C is the drug’s solubility (mg/mL). A volume of 250 mL, the typical volume of water consumed during human bioavailability studies, is too large to be appropriate to estimate the volume of water flush administered to a fasted dog.
Therefore, other volumes were explored for the calculation of a Do. In one analysis, we used a volume of 6 mL because this is often the volume administered to dogs with an oral medication (a single “flush” with a 6-mL syringe). A volume of 6 mL also has been suggested as the residual volume in the empty canine stomach (, ). Additional analysis was performed using 35 mL because it is equivalent to the 250 mL ( one cup) when scaled to the size of an average Beagle dog (the breed used most often in oral drug absorption studies). The relationships between canine estimates of F versus human BCS values, solubility, LogP, Do, and F values for humans were compared by linear regression. This was accomplished using the Proc Reg procedure in SAS (version 9.3).
Both slope and intercepts were included in the regression equation. Confidence and prediction intervals about the regression line were set at alpha = 0.10 (90% intervals, 5% in each tail). The regression of Do on F was expressed relative to the natural logarithm of Do (LD).
This evaluation was conducted both at Do values estimated at a volume of 6 mL (LD6) and 35 mL (LD35). RESULTS Oral absorption data were obtained for 50 drugs for which human and canine data were available (–; Papich, 1986, Pharmacokinetics of ranitidine and cimetidine in dogs, unpublished data; –; Papich, 1988, Pharmacokinetics of doxycycline in dogs after intravenous administration and oral administration of doxycycline hyclate and doxycycline monohydrate, unpublished data; –; Papich, 1986, Pharmacokinetics of lorazepam in dogs, unpublished data; –). Four drugs had conflicting data and therefore were listed twice to include both sets of data. For two drugs (, ), publications addressing BCS biowaivers for human formulations had data that conflicted with an official web site or other published data (–). These drugs were listed twice to accommodate both data.
Many of these drugs were administered to dogs as the human formulation or as a compounded product when there was no approved veterinary counterpart. For two drugs (furosemide and phenobarbital), two sets of canine values were considered because of duplication of published data. There was a relatively even distribution of drugs among the four BCS classes. There were 16 drugs from class I, 9 drugs from class II, 15 drugs from class III, and 10 drugs from class IV. As a percentage of drugs, this was a higher representation of class III and class IV drugs than the analysis of Takagi et al. Table summarizes the information used in our examination of the relationships between drug physicochemical parameters and the oral absorption performance in humans and dogs.
For consistency, we relied primarily upon two sources of information for the BCS classification: Kasim et al. , using their LogP-based estimates for our predictions of drug permeability, and Wu and Benet.
For those several compounds that were not contained within those two references, other published values were used (, ). Despite recognized diversity in some of the values across these various information sources, we concluded that since the magnitude of differences was small relative to the overall strength of the trends that were observed, any error that may have been introduced by the selection of publications would not influence the conclusions derived from our comparison. Do dose number (defined in the text), LogP log of octanol/water partition coefficient, F systemic bioavailability, BCS class Biopharmaceutical Classification System class as defined in the references cited in the text When assessing the relationship between estimates of F in dogs and humans (Fig. ), we observed that the slope significantly differed from zero ( P = 0.0055). However, the corresponding low coefficient of determination ( R 2 = 0.15) indicates that human estimates of F very poorly predicted canine F values. Without considering drug physicochemical characteristics, the potential contribution of drug solubility and/or permeability on the observed interspecies inconsistencies could be determined (and hence, the reason behind our further examination of these data). The intercept of the regression line was 0.31, which might be construed as suggesting a trend toward higher canine drug bioavailability when the human oral drug bioavailability is very low. Indeed, of the nine drugs with human estimates of F less than 0.40, six were associated with canine F values equal to or greater than that observed in humans.
However, given the very low R 2 value, such conclusions should be construed as an overinterpretation of the observed canine/human relationship. Along with this caution is the observation of wider confidence and predictions toward the intercept. The relationship between oral bioavailability ( F) observed in humans versus dogs. Although there was much variability in the relationship between estimated values of F in dogs and people, a statistically significant correlation was observed ( p = 0.0055). The confidence interval (as defined by the shaded region around the regression line) reflects variability both in slope and intercept attributable to the uncertainty in the estimated regression line. While the wider prediction interval likewise reflects the uncertainty of the data, it predicts the interval within which the regression will be contained (90% of the time) if the analysis were repeated using a comparable human and canine populations (i.e., relating the prediction of dog F from human F). The intercept (dog F observed when the human F = 0) is 0.31, indicating an overall trend toward a higher bioavailability in dogs at very small human values of F.
The slope of the regression of dog versus human is 0.42 Despite the low overall correlation between human and canine F values, when segregated according to its BCS class relationship, patterns begin to emerge (Fig. ). We observe that as compared to that observed in BCS classes III and IV, many of the compounds contained in BCS classes I and II demonstrated a good correlation between F in dogs and humans. In terms of the percent of the listed compounds where the human and canine F values differed by no more than +/−20%, these were found to be 79, 64, 36, and 20% for BCS classes I–IV, respectively (Table ). For BCS class I compounds, we observed a trend toward a lower bioavailability in dogs as compared to that in humans (Fig. ). Similarly, the class IV compounds tended to exhibit a comparatively lower oral bioavailability in dogs (Fig. ). For the nonsteroidal anti-inflammatory drugs (NSAIDs), all but one showed similar F values in dogs and humans (four were in class II or IV), which is consistent with their BCS classification of a low solubility compound being primarily a function of their behavior in acid.
A surprisingly high number of drugs in class II (Fig. ) showed similar or higher F values in dogs compared to humans. Several of these compounds were weak bases. Comparative F, dog versus human a BCS Drug Human F (dose mg) Dog F (dose mg) Therapeutic class p Ka First-pass loss Cyp enzyme Reference Similar 1 Bisoprolol 0.8 (10) 0.91 (11) Beta-1 adrenergic blocker 9.5 (base) No Cyp3A4, some Cyp2D6 DrugBank Similar 1 Chlorpheniramine 0.41 (4) 0.36 (100) Alkylamine antihistamine 9.2 (base) Yes CYP2D6; Similar 1 Clindamycin 0.87 (300) 0.73 (150) Lincosamide 7.7 (base) No CYP3A4; Wynalda et al. Similar 1 Digoxin 0.7 (0.25) 0.58 (0.36) Cardiotonic glycoside – No Pgp may limit absorption. BCS class Biopharmaceutical Classification System class as defined in the references cited in the text, F systemic bioavailability, Cyp enzyme cytochrome P450 enzyme, NSAID nonsteroidal anti-inflammatory drug aDefinition of similar versus not similar: “Similar” is as any human/canine value of F that differed by no more than +/−20%. The 20% value was based upon the traditional bioequivalence (BE) criteria (linear scale) of equivalence being +/−20%. “Not similar” was defined as outside this range As compared to that seen for BCS classes I and II, far fewer compounds exhibited comparable human versus dog bioavailability for BCS classes III and IV.
With regard to the latter two classes, there was no obvious pattern identified such that very high or very low oral absorption could be correlated with values of F when comparing dogs to humans. For example, for class III drugs (Fig. ), there was somewhat of an even distribution above and below a line of unity. There were examples of some compounds that were more bioavailable in humans than in dogs (e.g., codeine), while others were more bioavailable in dogs than humans (e.g., ranitidine). One of the potential sources of interspecies bias is the difference in gastric volume versus dose.
For this reason, we estimated the Do, using a volume of 6 or 35 mL (representing a range of volumes administered in studies where dogs are administered a water flush after oral dose administration). As shown in Fig. (6 mL volume) and b (35 mL volume), no obvious association between the calculated Do (expressed as LD) and F could be identified. This is evidenced both by low R 2 values (0.031 and 0.029 and for 35 mL (LD35) and 6 mL (LD6), respectively) and the lack of statistical significance when evaluating the slope of the regression line ( P = 0.23 and 0.22 for 3a and b, respectively), indicating that these slopes do not significantly differ from zero.
Furthermore, the similarity in width of the confidence and prediction intervals at the upper and lower portions of the profile suggests that the error about the regression line is similar across the range of LD values obtained in this study. The influence of the log-transformed dose number ( LD) on the estimated value of F in dogs. A The relationship defined by volume of 6 mL (LD6). B The relationship defined by a volume of 35 mL (LD35). Interpretation of confidence versus prediction intervals corresponds with that previously described for Fig.
Figure illustrates that regardless of whether a volume of 6 or 35 mL was used in the analysis, there was little influence of fluid volume on Do above, or below, 1.0. This indicates that either volume could adequately reflect oral dose solubility as a function of administered dose for most drugs administered to dogs. Using a Do of 1.0 as the cutoff between low and high solubility (as used for the human BCS), only seven drugs (gabapentin, cimetidine, amlodipine, ciprofloxacin, sildenafil, theophylline, and atenolol) would be classified as high solubility (Do 1) using a volume of 6 mL. The influence of the lipophilicity (expressed as LogP) on F was examined in Fig. When considering all of the drugs included in this analysis, no association could be identified between LogP and F ( R 2 = 0.0027). The large P value for the slope of the regression line ( P = 0.72) indicates that the slope defining the regression of LogP on canine F is not significantly different from zero. Furthermore, similarities in the width of the confidence and prediction intervals at the upper and lower portions of the profile suggest that that the error about the regression line was similar across the range of LogP values.
However, limiting our assessments to those drugs with F 0.80 (Fig. ), most had LogP values within the range of 0–4, suggesting transcellular absorption. The only compounds showing high F values but LogP.
Bcs Class
LogP value versus F value in dogs for 50 drugs. LogP is the experimentally determined lipid partition coefficient; the F value is the bioavailability in dogs. The estimated canine F value when LogP is zero is 0.62. The corresponding slope of the line is −0.008.
The large P value indicates that this is not a significant correlation, and therefore, the negative slope should not be construed as being indicative of any true relationship. Interpretation of confidence versus prediction intervals corresponds with that previously described for Fig. High bioavailability (approximately 80%) drugs in dogs compared to dose number and LogP. (Dose number and LogP were previously defined.) Four drugs (shown with open diamonds on the left side of the figure) were exceptions because they had LogP. DISCUSSION Whether we are providing values for F, solubility, permeability, or BCS classification, all values need to be viewed from the perspective as estimates derived under a specific set of experimental conditions. Thus, there are occasions where, for example, a compound was estimated as being within one BCS classification in one reference but as a different class for another reference (e.g., ciprofloxacin).
The tables available from Takagi et al. show some of these discrepancies. Similarly, values of F reported within this manuscript reflect a distinct set of experimental conditions, and therefore, there can be differences in reported estimates elsewhere, depending upon study conditions and population.
For example, we can consider the compound amlodipine. In one study, the human oral bioavailability was stated to be 63%.
However, in the Pfizer monograph for amlodipine besylate , the absolute bioavailability of an oral administration ranged between 64 and 90%. Accordingly, on an individual compound basis, the estimated ratio of dog and human F values provided in this manuscript should be considered a single point within a distribution of potential estimates. That said, when viewed across a diverse library of compounds (as we have done in this manuscript), these values provide a tool for examining variables that can be used to examine interspecies relationships in drug absorption. Highly soluble and highly permeable compounds will likely exhibit similar oral drug bioavailabilities unless the drug exhibits differing extent of first-pass metabolism in dogs versus humans. Nevertheless, we cannot conclude that a drug’s BCS classification will necessarily translate across species.
BCS solubility classifications are traditionally based upon a calculation of the Do, which assumes a dissolution volume of 250 mL (approximately one cup of water). That volume is far greater than the gastric volume of the fasted dog.
Typically, studies performed in dogs will include a water flush of 6 or 12 mL because, for practical reasons, these are common plastic syringe sizes. We included a high value of 35 mL in our analysis because this represents the equivalent to the 250 mL ( one cup) in humans scaled to the size of an average Beagle dog (the breed used most often in oral drug absorption studies). Accordingly, compounds considered highly soluble in a human population may not meet the criteria for highly soluble in dogs. Regardless of whether we consider the canine gastric volume to be 6 or 35 mL, we found that the solubility classification would be affected for only a few compounds (Fig. ).
Using a Do cutoff of 1.0 for distinguishing between low or high solubility compounds, only seven drugs, gabapentin, cimetidine, amlodipine, ciprofloxacin, sildenafil, theophylline, and atenolol, would have had a different solubility classification as a function of different volumes used for these calculations. In some cases, this solubility difference may impact oral drug absorption. For example, in the case of ciprofloxacin, Papich observed that differences in the volume administered with the oral dose may indeed affect F in dogs. When ciprofloxacin tablets were administered with a volume that decreased the Do below 1, oral absorption was better than when the Do was 1. This observation points to the importance of dosing regimens and study design when interpreting study data.
Another challenge facing interspecies BCS extrapolations is that by convention, a drug is classified as highly soluble based upon the highest administered dose. In the case of diazepam, the drug itself is poorly soluble, but may be listed as a human BCS class I compound because of the low administered dose. The importance of considering dose was underscored with diazepam where our cited canine investigations used a dose of 40 mg (i.e., approximately 4 mg/kg). This is in contrast with a 10-mg dose in humans (i.e., approximately 0.14 mg/kg for a 70-kg person). Thus, although included in the list of BCS class I compound for this analysis, it would have been more appropriate to have classified diazepam as a BCS class II drug (at least for the sake of the canine investigation). Diazepam was in the lower right quadrant of the group of class I drugs (Fig. ), and considering the low solubility of diazepam, it is not surprising that its oral bioavailability was very poor in dogs where tablet dissolution is further compromised by a rapid GI transit time ( vide infra).
The BCS classification is influenced by the ability of the highest dose strength to be fully solubilized in 250 mL ( one cup) of aqueous media over the pH range of 1–7.5. However, for low solubility compounds, interspecies differences in absorbable dose may also be impacted by the composition of the GI fluids.
In this regard, the unique composition of canine GI fluid can lead to differences in canine-human in vivo tablet dissolution. Because dissolution impacts oral bioavailability, it is important to distinguish between criteria used to evaluate the inherent solubility of the active pharmaceutical ingredient (API) versus that used to characterize in vivo dissolution of a dosage form. D R = A × D h × C s − X d V These considerations will be particularly important for low solubility compounds and may further complicate efforts to generate interspecies comparisons based upon the bioavailability of solid oral dosage forms. Another challenge facing an examination of canine BCS criteria is that of defining what constitutes a high versus low permeability compound.
Because there are no commercially available canine cell lines , in the absence of in vitro permeability data, we have relied upon LogP values as the basis for estimating a canine BCS permeability cutoff value. Our selection of LogP values was founded upon published reports where LogP was used as a surrogate for human permeability studies (–). These referenced studies used metoprolol, with a LogP = 1.72 as the reference standard for the cutoff between high/low permeability. Drugs with LogP 1.72 are classified as highly permeable, while those with LogP 0.90 (amoxicillin and cephalexin). Despite their poor membrane permeability, both compounds undergo active transport via peptide transporter 1 (PEPT1) (–).
Thus, enterocyte penetration via facilitated/active rather than passive processes will result in an extent of absorption that cannot be predicted solely on the basis of BCS classification criteria. Moreover, numerous BCS class III and IV compounds were associated with an F 0.80 in dogs but not in humans. It is undetermined why these drugs defined as poorly permeable exhibited such high F values in dogs. Because the dog intestine is anatomically shorter than that of humans, in order to absorb nutrients, the canine intestine may have evolved a greater capacity for intestinal uptake transport as compared to that of humans. Less active intestinal efflux transport also is possible.
For low molecular weight compounds, there is also greater paracellular absorption for some compounds in dogs than humans ( vide infra). For furosemide, there was higher bioavailability from a 400-mg dose ( F 0.77) compared to a tenfold lower dose of 40 mg ( F 0.38). Class III and IV drugs ordinarily typically do not exhibit saturable drug efflux. But for this class IV compound, efflux transport apparently can be saturated at high doses, thus increasing its oral bioavailability (–).
Therefore, it would seem likely that at the high doses of furosemide administered in the canine study, an increase in enterocyte concentrations produced a saturation of efflux transporters and a corresponding larger F. When drugs with relatively high F values in dogs were considered (Fig. ), most of those compounds were associated with LogP values 0. The only drugs in this study that showed both high F values in dogs and LogP. CONCLUSION This investigation showed that applying the same BCS criteria to dogs and humans can be problematic. At least in part, when attempting to designate a BCS classification for dogs, there is a need to develop canine-specific solubility and permeability assessments. Ultimately, even when the necessary in vitro methods for estimating canine drug solubility and permeability have been developed, canine-human physiological differences can result in marked differences in systemic absorption due to transporter functions, drug metabolism, and the leakier canine intestinal membranes.
Clearly, there remains much work to be done in order to improve our ability to predict drug absorption in the dog when based upon preliminary drug physicochemical characterization and an interspecies extrapolation of in vivo PK information. Efforts to predict drug absorption (or to understand the causative factors impacting interspecies differences in F) is dependent upon a wide array of variables including API solubility and permeability (BCS), formulation factors, and physiological variables (including regional permeability differences, which could differ between species), GI pH, luminal and mucosal enzymology, and intestinal motility, first-pass drug metabolism, and transporter activity (–). It is for this reason that a blending of BCS and the BDDCS may provide far better predictions of canine versus human drug absorption characteristics than would either classification system alone. Comparing drug absorption characteristics in dogs and humans, while important for interspecies extrapolations and for formulation development, also provides valuable insights into the variables that can influence drug absorption (and interindividual differences) that can occur in the presence of human or canine GI pathologies, breed potential breed effects on canine drug absorption, or the changes in drug absorption that may occur in the geriatric dog or human population. Such predictions are predicated largely upon the development of mechanistic ( in silico) models. By understanding the impact of these critical variables on the rate and extent of drug absorption, these models can be positioned for use in predicting the canine to human (and vice versa) differences in oral drug absorption and in vivo product performance. Our ongoing efforts are based upon the use of this drug list to explore published human-canine differences in drug metabolism, along with an evaluation of other potential variables.
We anticipate that through these efforts, we will obtain a better appreciation of the pivotal factors dictating in vivo product absorption and determine potential sources of error when attempting to extrapolate information obtained in humans to support in silico predictions of in vivo drug absorption characteristics in dogs. At the time this manuscript was prepared, we did not have access to the paper published by Musther et al. Their paper included 125 paired datasets comparing F between dogs and humans and included many of the same studies that were used in our analysis.
As we report here, they also found a poor correlation in F for drugs between dogs and humans with an R value of 0.37. Musther et al. did not include BCS criteria in their analysis, but instead, separated compounds into acidic, basic, neutral, or zwitterions. This improved the prediction slightly for acidic drugs, some of which were likely BCS class II compounds. A prediction model of human F from canine values, based on a linear regression model, resulted in wide prediction intervals with a low concordance correlation coefficient (precision of the prediction), confirming the lack of agreement between human and dog F values. As in our studies, they found that Beagle dogs were represented more often than other dog breeds for bioavailability determination in most studies (66%).
We encountered similar problems as described by Musther et al. when extracting data from published studies. Some studies provided full details of the methods and results and others provided limited data. They concluded, as we did, that metabolic differences between species could play a more important role in defining disparities between human and animal drug bioavailability.
The “” is an FDA guidance document, which allows pharmaceutical companies to forego clinical bioequivalence studies, if their drug product meets the specification detailed in the guidance. The principles of the BCS classification system can be applied to NDA and ANDA approvals as well as to scale-up and post approval changes in drug manufacturing.
Bcs Class 2 Drugs List
A waiver of In-vivo Bioavailability and Biioequivalence studies based on the BCS classification can therefore save pharmaceutical companies a significant amount of development time and reduce development costs. The BCS classification system is based on the scientific rationale that, if the highest dose of a drug candidate is readily soluble in the average fluid volume present in the stomach (250 ml) and the drug is more than 85% absorbed, then the in vitro drug product dissolution profiles should allow assessment of the equivalence of different drug formulations. Solubility and dissolution can be easily measured in vitro. Extent of absorption has historically been determined by conducting mass balance studies both preclinically and clinically.
However, our work and that of our collaborators has demonstrated that the effective intestinal permeability (Peff) of therapeutic agents correlates well with total fraction absorbed in both humans, rats, and to a lesser extent in vitro tissue culture systems (1-5). Based on these studies a drug candidate can fall into one of four BCS categories, with category I, High Permeability and High Solubility, being the subject of the BCS guidance.
Bcs Classification Of Drugs List Who
The WHO has recently recommended biowaivers for Class III and some Class II drugs and AAPS-FDA scientific conferences have recommended biowaivers for Class III compounds as well.